- Apr 2, 2009
- 5,171
- 13,288
- 66
The FDA has just issued an Advanced Notice of Proposed Rulemaking (ANPRM) for e-liquid and/or e-cig packaging and labeling.
Nicotine Exposure Warnings and Child-Resistant Packaging for Liquid Nicotine, Nicotine-Containing E-Liquid(s), and Other Tobacco Products
http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM453226.pdf
This same docket was exposed and discussed on another ECF thread at
Interesting new docket opened and withdrawn | E-Cigarette Forum
Although FDA doesn't have any legal authority to formally propose or impose any additional regulations on nicotine vapor products unless and until the Final Rule for the FDA deeming regulation is approved by OMB and printed in the Federal Register (which could take several more months), the agency issued this ANPRM to generate more fear mongering news stories about the negligible risks of e-liquid, to further lobby for the deeming regulation, and to further deceive the public and the news media to believe that the deeming regulation is a done deal.
Note that FDA's ANPRM is requesting comments about color graphic warnings for e-liquid and/or all e-cigs. But the FDA still hasn't proposed new regulations (that Congress mandated the FDA impose in 2009) for color graphic warnings on cigarette packs (since Judge Leon ruled that FDA's previous cigarette warnings were unconstitutional because they misrepresented some smoking risks, demonized smoking and stigmatized smokers, and included the phone number 1-800-Quit-Now that hawked FDA approved drugs as the only effective way to quit smoking).
Nicotine Exposure Warnings and Child-Resistant Packaging for Liquid Nicotine, Nicotine-Containing E-Liquid(s), and Other Tobacco Products
http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM453226.pdf
This same docket was exposed and discussed on another ECF thread at
Interesting new docket opened and withdrawn | E-Cigarette Forum
Although FDA doesn't have any legal authority to formally propose or impose any additional regulations on nicotine vapor products unless and until the Final Rule for the FDA deeming regulation is approved by OMB and printed in the Federal Register (which could take several more months), the agency issued this ANPRM to generate more fear mongering news stories about the negligible risks of e-liquid, to further lobby for the deeming regulation, and to further deceive the public and the news media to believe that the deeming regulation is a done deal.
Note that FDA's ANPRM is requesting comments about color graphic warnings for e-liquid and/or all e-cigs. But the FDA still hasn't proposed new regulations (that Congress mandated the FDA impose in 2009) for color graphic warnings on cigarette packs (since Judge Leon ruled that FDA's previous cigarette warnings were unconstitutional because they misrepresented some smoking risks, demonized smoking and stigmatized smokers, and included the phone number 1-800-Quit-Now that hawked FDA approved drugs as the only effective way to quit smoking).


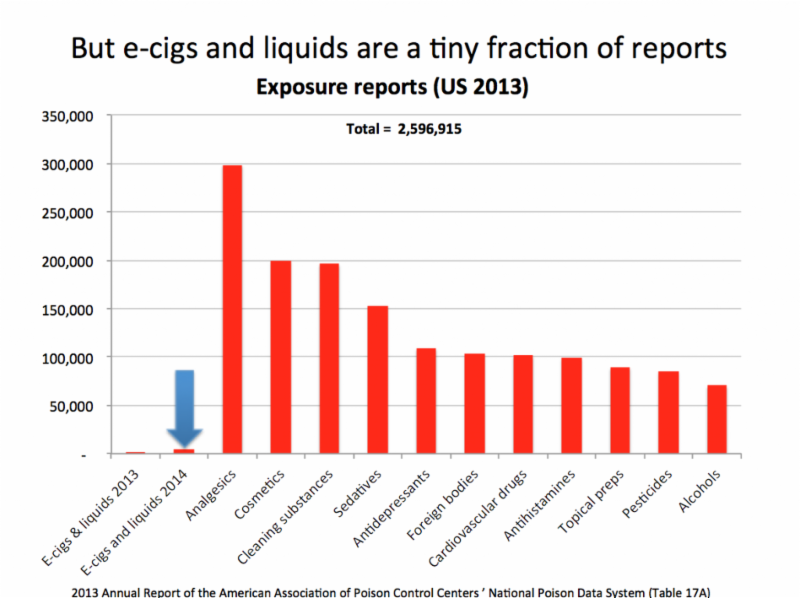

 ) - that have been pushed by the nannystaters, and the activists who can't really stop a product they don't like, but want to 'make the company pay' through really stupid labels and finally, out of necessity, grudgingly accepted by the companies, who are more afraid of the trial lawyers than the FDA.
) - that have been pushed by the nannystaters, and the activists who can't really stop a product they don't like, but want to 'make the company pay' through really stupid labels and finally, out of necessity, grudgingly accepted by the companies, who are more afraid of the trial lawyers than the FDA.