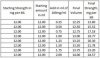
I have a bunch of free sample juice from MBV and I was wondering if anyone had an easy formula for increasing or decreasing the nic to a certain level.
Heres an example: I have a 15ml bottle at 12mg strength. I'm guessing out of that 15ml bottle there is actually 10-12ml left in it after sampling and what not. My desired nic level is either 18 or 24. I have a jug of 100mg nic so I'm wondering how much I should add. This sounds like a word problem from middle school haha.
I'm not looking for how much to add, just a formula for how to figure it out. I'm sure there will be other circumstances where people will want to change nic levels but not have the exact ml/mg juice. Thanks in advance for your help!!!
Heres an example: I have a 15ml bottle at 12mg strength. I'm guessing out of that 15ml bottle there is actually 10-12ml left in it after sampling and what not. My desired nic level is either 18 or 24. I have a jug of 100mg nic so I'm wondering how much I should add. This sounds like a word problem from middle school haha.
I'm not looking for how much to add, just a formula for how to figure it out. I'm sure there will be other circumstances where people will want to change nic levels but not have the exact ml/mg juice. Thanks in advance for your help!!!