You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Vinegar?
- Thread starter pinellaspete
- Start date
- th_trl_thread_readers 0
-
- Tags
- ecigexpress halo madvapes vapor pro
- Status
- Not open for further replies.
I wonder how this improves the characteristics of ejuice
Benzyl benzoate - Wikipedia, the free encyclopedia
http://www.ebay.com/itm/20ML-Benzyl...950?pt=LH_DefaultDomain_0&hash=item3a692d5dae
Also why in the world would this be in ecigs
METHYL OCTANOATE
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. Substance may be transported hot. If molten aluminum is involved, refer to GUIDE 169. (ERG, 2008)
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. (ERG, 2008)
Reactivity Profile
METHYL OCTANOATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
http://cameochemicals.noaa.gov/chemical/17404
Benzyl benzoate - Wikipedia, the free encyclopedia
http://www.ebay.com/itm/20ML-Benzyl...950?pt=LH_DefaultDomain_0&hash=item3a692d5dae
Also why in the world would this be in ecigs
METHYL OCTANOATE
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. Substance may be transported hot. If molten aluminum is involved, refer to GUIDE 169. (ERG, 2008)
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. (ERG, 2008)
Reactivity Profile
METHYL OCTANOATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
http://cameochemicals.noaa.gov/chemical/17404
Last edited:
Vinegar is typically around 3 - 5% acetic acid by weight, I would start with that. Use that mixture drop by drop in your juices.
In a 5ml bottle that is probably not more than 1/8 - 1/4 teaspoon.
In a 5ml bottle that is probably not more than 1/8 - 1/4 teaspoon.
I wonder if this will work as it has all three acids
http://www.ebay.com/itm/Acid-Blend-...139?pt=LH_DefaultDomain_0&hash=item2317f78a5b
I was also thinking , MTS Vape wizard is super dark. I wonder if that is mainly caramel color .
http://www.ebay.com/itm/Acid-Blend-...139?pt=LH_DefaultDomain_0&hash=item2317f78a5b
I was also thinking , MTS Vape wizard is super dark. I wonder if that is mainly caramel color .
Last edited:
Srt8V8ping,
Wow that's a great find! I did some research on those acids and you can buy them individually. Each acid has its own distinct base flavor so we might not want to use all of them in the same mix.
Okay...I think we need to use at least one of the following in each and every one of our mixes to lower the PH:
Citric Acid
Lemon Juice
Vinegar
Malic Acid
Tartaric Acid
Caramel Color
I think you would pick your acid depending on the main mix flavor so that the acid flavor would compliment the main flavor. Use Lemon Juice or Tartaric Acid with fruit flavors? Use Caramel Color with RY4 and tobacco flavors?
Okay...I will be mixing a batch of juices tonight. Does anyone have ideas as to what a good test of this concept might be?
Pete
Wow that's a great find! I did some research on those acids and you can buy them individually. Each acid has its own distinct base flavor so we might not want to use all of them in the same mix.
Okay...I think we need to use at least one of the following in each and every one of our mixes to lower the PH:
Citric Acid
Lemon Juice
Vinegar
Malic Acid
Tartaric Acid
Caramel Color
I think you would pick your acid depending on the main mix flavor so that the acid flavor would compliment the main flavor. Use Lemon Juice or Tartaric Acid with fruit flavors? Use Caramel Color with RY4 and tobacco flavors?
Okay...I will be mixing a batch of juices tonight. Does anyone have ideas as to what a good test of this concept might be?
Pete
Srt8V8ping,
About the Vape Wizard...
The Vape Wizard is dark in color but they also state that it does NOT change the PH. If Caramel Color was used it would definitely lower the PH.
Pete
About the Vape Wizard...
The Vape Wizard is dark in color but they also state that it does NOT change the PH. If Caramel Color was used it would definitely lower the PH.
Pete
Hmmm.... have to look some more into this. Maybe when I make a batch of juice up will split it into two bottles and see if there is a marked difference with using vinegar.
Not really able to tell that much of a difference with this DIY RY4, took about 4-5ml and added maybe 4 or 5 drops of white vinegar to it.
Not really able to tell that much of a difference with this DIY RY4, took about 4-5ml and added maybe 4 or 5 drops of white vinegar to it.
Hmmm.... have to look some more into this. Maybe when I make a batch of juice up will split it into two bottles and see if there is a marked difference with using vinegar.
Not really able to tell that much of a difference with this DIY RY4, took about 4-5ml and added maybe 4 or 5 drops of white vinegar to it.
That brings up another question...use distilled white or cider vinegar?
the difference in the acidity shouldn't make that much of a difference, not like we're mixing gallons of it here.
No, but there's a marked difference in taste between the two, imo. Even if you don't actually taste the vinegar itself, I'm wondering if the composition of one would have a different effect on juice than the other?
Almost all the vinegar I have seen for sale has printed on the label "Diluted with water to 5% acidity." It didn't matter whether it was distilled, apple cider, red wine or balsamic vinegar, they all had been diluted with water to 5% acidity. I think this is done to insure uniform acidity levels when it is used in recipes. They do however all taste different, and I am sure the vinegar flavor would affect the final juice flavor.
Pete
Pete
Iv tried app;e cider vinegar and white vinegar in a two 4ml recipes and i didnt taste either . It does seem to make the flavors come a bit more a live but not a huge difference . I put 3 drops in each .
Here's some interesting information about PH:
Properties of Vinegar - pH of Vinegar
The term "pH" is derived from "potential hydrogen" and refers to the amount of hydrogen ions present in solution.
Mathematically, pH is equal to the negative logarithm (base 10) of the hydrogen ion concentration in moles per liter, so if the pH of a solution decreases by 1 pH unit then its hydrogen ion concentration increases by ten times.
Pure water has a pH of 7 and is neutral whereas anything with a pH less than 7 is acidic and anything with a pH greater than 7 is basic.
The pH of vinegar depends upon how much acid is present, but most commercial distilled white vinegars contain 5% acetic acid and have a pH of about 2.4.
To put that in perspective, the following table compares the pH of vinegar to some other common solutions:
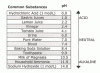
(Sorry about the small size. If you click once on the image it will enlarge.)
Pete
Properties of Vinegar - pH of Vinegar
The term "pH" is derived from "potential hydrogen" and refers to the amount of hydrogen ions present in solution.
Mathematically, pH is equal to the negative logarithm (base 10) of the hydrogen ion concentration in moles per liter, so if the pH of a solution decreases by 1 pH unit then its hydrogen ion concentration increases by ten times.
Pure water has a pH of 7 and is neutral whereas anything with a pH less than 7 is acidic and anything with a pH greater than 7 is basic.
The pH of vinegar depends upon how much acid is present, but most commercial distilled white vinegars contain 5% acetic acid and have a pH of about 2.4.
To put that in perspective, the following table compares the pH of vinegar to some other common solutions:

(Sorry about the small size. If you click once on the image it will enlarge.)
Pete
Iv tried app;e cider vinegar and white vinegar in a two 4ml recipes and i didnt taste either . It does seem to make the flavors come a bit more a live but not a huge difference . I put 3 drops in each .
The few drops I put in didn't seem to effect the taste or give me the 'vinegar' taste.
I'm half tempted to go get an orange or a lemon and try a few drops of juice. The problem I am thinking of there would end up being the extra sugar gumming up the heating coils.
I'm also thinking that the sugars would have an effect on the taste as well as the pH of the juice.
I put some concentrated lemon flavor in my tobacco juice,I didn't taste the lemon and it made the juice taste better.But I am worried that it will damage the cartomizer.
brokepainter,
I think it may actually be good for your atomizer. Citric acid is a natural chelating agent. It is used to remove scale from boilers and evaporators according to Wikipedia.
Citrus juices are excellent natural cleaners.
Pete
I think it may actually be good for your atomizer. Citric acid is a natural chelating agent. It is used to remove scale from boilers and evaporators according to Wikipedia.
Citrus juices are excellent natural cleaners.
Pete
You can buy citric acid as a powder and mix with pg. I use occasionallyThe few drops I put in didn't seem to effect the taste or give me the 'vinegar' taste.I'm half tempted to go get an orange or a lemon and try a few drops of juice. The problem I am thinking of there would end up being the extra sugar gumming up the heating coils.I'm also thinking that the sugars would have an effect on the taste as well as the pH of the juice.
You can buy citric acid as a powder and mix with pg. I use occasionally
At what ratio?
- Status
- Not open for further replies.
Similar threads
- Replies
- 0
- Views
- 501
- Replies
- 5
- Views
- 3K
Users who are viewing this thread
Total: 3 (members: 0, guests: 3)
