Turns out I have 2 gals of VG and 1/2 gal of PG. And about a gallon of undiluted nic.Only a gallon of each?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
New studies find carcinogens in vg and pg at high temps, even in tootle puffers
- Thread starter mikepetro
- Start date
- th_trl_thread_readers 0
- Status
- Not open for further replies.
The whole purpose to the test is to see if I see a correlation between boiling point and real life vaping.I'm not quite sure the boiling point tests will be all that useful or easy to do. First we have a number of charts showing the boiling point of various mixes and secondly "usable amount of vapor" is highly dependent on air flow.
Case in point: with a freshly wound coil and primed wick left to the open air for inspection hitting the fire button might produce little to no vapor however if you blow on the coil all sorts of vapor can be produced.
Just a thought.
The only boiling charts I have seen were mixed with water, I agree, that doesnt tell us much about our juice. I havent seen any charts that show the BP of various ratios of PG/VG mixed together.
Yes, "meaningful vapor" will be a subjective determination made by me, and may not extrapolate to other attys. I will take care to puff consistently across the tests. It should tell me if lowering the boiling point of the juice allows me to lower my atty temp or not.
Last edited:
If testing the boiling point is not extremely arduous, I will make a chart of the BP of PG/VG ratios (no nic) at 10% increments. This might be a good reference to have in that I have never seen it elsewhere.I've seen charts of VG/water mixes and PG/water mixes, but I've never seen one of PG/VG mixes, have you?
Appreciate the offer, but I think I have enough to easily get though these tests.I'd be willing to contribute an entire Glycube to this. However it probably wouldn't appear on your doorstep until next week sometime.
Turns out I have 2 gals of VG and 1/2 gal of PG. And about a gallon of undiluted nic.
That sounds more like the Mike I've come to know

To keep from losing the work I put into temp measurements, I have compiled it all into a blog entry. Otherwise it might get lost in the ebb and flow of the thread,
Real Life temperature measurements inside a 2016 era atty | E-Cigarette Forum
I will do another blog with all of the Boiling Point stuff once its done.
Real Life temperature measurements inside a 2016 era atty | E-Cigarette Forum
I will do another blog with all of the Boiling Point stuff once its done.
I'm not quite sure the boiling point tests will be all that useful or easy to do. First we have a number of charts showing the boiling point of various mixes and secondly "usable amount of vapor" is highly dependent on air flow.
Case in point: with a freshly wound coil and primed wick left to the open air for inspection hitting the fire button might produce little to no vapor however if you blow on the coil all sorts of vapor can be produced.
Just a thought.
Knowing the Boiling Point of VG and or PG is Good. But do we know the Boiling Point of VG/Water/Nic? Or PG/Nic? Or as Rossum mentioned, VG/PG? And what effect might Flavorings/Sweeteners have on a Boiling Point?
Testing to "Known's" is also a form of Calibration/Verification of the testing equipment that is being Used.
I am not even going to touch flavorings......Knowing the Boiling Point of VG and or PG is Good. But do we know the Boiling Point of VG/Water/Nic? Or PG/Nic? Or as Rossum mentioned, VG/PG? And what effect might Flavorings/Sweeteners have on a Boiling Point?
Testing to "Known's" is also a form of Calibration/Verification of the testing equipment that is being Used.
I am not even going to touch flavorings......

Yeah... A lot of Bad Things came Flying out of Pandora's Box (Jar) when it was Opened.
But the Last Thing inside gave Us the ability to deal with them. If we used it.
I am just intimidated by the number of variables, aint no way in hell I am going to volunteer to go down that road.
I am just intimidated by the number of variables, aint no way in hell I am going to volunteer to go down that road.
For the Average Flavoring being made up mainly of PG, it would be probably be Safe to Say that the addition of Flavoring should Lower the boiling point of a e-Liquid that contains VG.
How much would of course be Dependent on the Percentage of the Flavoring used.
This would also seem to Validate the reason for Testing a VG/PG solution.
Because a 70 ~ 75% VG/Nic to PG solution would probably Closely reflect what the Boiling Point of what a High VG Flavored e-Liquid would be.
Last edited:
In most cases I would equate them to pg in terms of BP, as a guess. Now if they contain alcohol it would have an even greater effect. Bottom line is we will never know as flavor manufacturers dont post that data.For the Average Flavoring being made up mainly of PG, it would be probably be Safe to Say that the addition of Flavoring should Lower the boiling point of a e-Liquid that contains VG.
ETA: all of the testing I am doing is without any flavorings or other additives. Just PG/VG/Nic/H20
In thinking about it, I wonder how accurate of a test I can do of the Boilng Point of a mixture that contains H20. Would the water possibly evaporate off before the mixture boiled? Mainly concerned about the 90% VG 10% distilled water test.
It is hard to mimic the closed chamber environment that we use versus an open container, particularly when we hit it with a high temperature rather immediately versus coming to a boil.
It's always nice to introduce another variable lol!
It's always nice to introduce another variable lol!
In thinking about it, I wonder how accurate of a test I can do of the Boilng Point of a mixture that contains H20. Would the water possibly evaporate off before the mixture boiled? Mainly concerned about the 90% VG 10% distilled water test.
If VG/Water is a Homogeneous Solution (which I believe it is), then I don't think the Water would Boil Off before the VG Boils.
If my measurement differs greatly from the VG/H20 chart, I will dig further.If VG/Water is a Homogeneous Solution (which I believe it is), then I don't think the Water would Boil Off before the VG Boils.
Not sure about that. Back when I used to run a certain vendor's 35PG/65VG house juice in iClear 16 and iClear 30s "tanks", I would add a bit of DW to the juice to thin it and make it wick better, but as a tank got close to empty, it seemed that what was left had a higher viscosity than what I started with. This implied to me that the water did boil off more quickly than the other constituents (particularly the VG).If VG/Water is a Homogeneous Solution (which I believe it is), then I don't think the Water would Boil Off before the VG Boils.
That said, I think some kind of PG/VG boiling point curve would be really good data to have, and I can fully understand why Mike doesn't want to add more variables.
Not sure about that. Back when I used to run a certain vendor's 35PG/65VG house juice in iClear 16 and iClear 30s "tanks", I would add a bit of DW to the juice to thin it and make it wick better, but as a tank got close to empty, it seemed that what was left had a higher viscosity than what I started with. This implied to me that the water did boil off more quickly than the other constituents (particularly the VG).
That said, I think some kind of PG/VG boiling point curve would be really good data to have, and I can fully understand why Mike doesn't want to add more variables.
I could be. It could be that the Water Boils Off in a Non-Linear fashion.
Meaning that the Rate at which the Water Boils Off is Greater than the Rate the VG Boils Off at. Verses the Water Boiling Off 1st then the VG Boiling Off next in a Stair Step fashion.
The More I think about it, you are probably right.
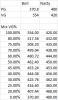
Here's some "theoretical" data we can compare against the actual measurements forthcoming.
I took the temps we have for the pure PG and VG boiling temps and interpolated temps from the mix ratios between them. I also did the same for our temps where nasties are formed from data posted here.
So this assumes that VG will boil at 554F with nasties at 420F and PG at 370/480F and so if you have a 50/50 mix you'll boil at 462F with the nasties at 450F. So these are numbers to test if it is fair to do linear interpolation for the mix temps or if something else is going on. There may be no truth to this whatsoever. The measurements will tell us about the boiling temps but we still don't know about the nasty formations. It could be that nasties still start at the lower 420F bound set by the VG or it could be that the VG is whisked away at a lower temp by the PG in the mix.
The Margin shown below is how many degrees of headroom we have in the interpolated values. It is interesting to note that by this we need at least 55% PG to stay in safe zone. In reality there is nic and flavoring that will add PG and/or water/other content as well.
Here's the table:

I took the temps we have for the pure PG and VG boiling temps and interpolated temps from the mix ratios between them. I also did the same for our temps where nasties are formed from data posted here.
So this assumes that VG will boil at 554F with nasties at 420F and PG at 370/480F and so if you have a 50/50 mix you'll boil at 462F with the nasties at 450F. So these are numbers to test if it is fair to do linear interpolation for the mix temps or if something else is going on. There may be no truth to this whatsoever. The measurements will tell us about the boiling temps but we still don't know about the nasty formations. It could be that nasties still start at the lower 420F bound set by the VG or it could be that the VG is whisked away at a lower temp by the PG in the mix.
The Margin shown below is how many degrees of headroom we have in the interpolated values. It is interesting to note that by this we need at least 55% PG to stay in safe zone. In reality there is nic and flavoring that will add PG and/or water/other content as well.
Here's the table:
Attachments
This is the Wang Study of a 50/50 mix, it shows the nasties starting to develop at ~410F.

- Status
- Not open for further replies.
Similar threads
- Replies
- 11
- Views
- 9K
- Replies
- 92
- Views
- 15K
- Replies
- 5
- Views
- 4K
- Locked
- Replies
- 33
- Views
- 2K
- Replies
- 68
- Views
- 12K
Users who are viewing this thread
Total: 4 (members: 0, guests: 4)