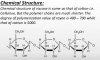
Let get down to technicals here.
Rayon is a chemically refined, reformed and purified cellulose based polymer. Many have been lauding it as the next big thing for e-cigarettes. I have personally ordered a sh*t ton of it and I am eager to see if it is as good as reported. I only got about a few pages into the main thread, but I didn't see much technical details about why it is good.
What is rayon?
So both cotton and rayon are cellulose based polymers. As seen in the rayon thread, they have the same basic structure. Celluse is a polymer (like plastic, rubber and proteins).
Like other biological polymers, cellulose is made of monomer units. These are called D-Glucose. You can buy this monomer in the supermarket; its called dextrose. Proteins have various amino acid monomers. Think of monomers as lego blocks for building (the polymer); a big lego tower.
Cellulose has many d-glucose units linked end to end. These form cellulose chains. However, the complex chemical treatment process used for rayon changes things. It breaks longer cellulose chains into shorter elements. These can then be reformed using chemical treatment to form longer or shorter fibres, depending on the application.
How is Rayon made?
Rayon was first commercialized around 1905 as a cotton replacement. Whats wrong with cotton you ask? Rayon can be produced from cheaper precursor materials (eg wood and plant wastes) and specifically tailored for softness, strength and durability depending on application.
I will outline the modern rayon production method briefly. Basically, cellulose fibres are dissolved in chemicals, then pumped through thin jets into a chemical bath where they precipitate as fibres. These fibres are washed, dried, and stretched to straighten them.
1. Wood pulp sourced cellulose is partially dissolved in sodium hydroxide (caustic soda). This does not break the cellulose into its monomer glucose,but breaks up long cellulose monomer chains. Think of this as a chemical treatment to "break up" the fluffy, long filaments of the raw cellulose.
2. The resulting, shorter chain cellulose mush is pressed, dried and crumbled into white cellulose "crumbs". The crumbs are aged in air to help with the de-polymerization of long cellulose chains.
3. The alkali cellulose crumbs are reacted with carbon disulfide (this stuff is dangerously flammable) to form cellulose xanthate. This further de-polymerises the cellulose monomer chains. This carbon disulfide step makes the cellulose even more water soluble by forming soluble xanthate groups on cellulose monomer chains.
4. The cellulose xanathate is aged which further depolymerizes the long cellulose chains. The result is a solution of Viscose, where all the cellulose is fully dissolved and no solids remain.
5. The viscose solution is then pumped through a shower-head like "spinneret" and into sulfuric acid solution, where the dissolved smaller cellulose chains instantly solidify into fibres of rayon.
As can be seen in ray2.PNG(from the main rayon thread on forum), the basic monomer construction of rayon is identical to cellulose. Ok, so if their cellulose chains are identical, then why is rayon so much better??
Why is rayon better?
I have some ideas to explain this:
1. Rayon has a more organized polymer structure because the cellulose in it was reformed into desirable fibre lengths. Think of comparing a big jumbled mess of cotton string to a braided cotton rope made of many cotton twine strands. A cotton rope will soak up more water and remain saturated better than a disorganised cotton string.
2. Rayon is more chemically pure than cotton. Solid cellulose from wood fibres or plants is completely dissolved to form a solid-less solution. This provides a very high purity rayon fibre as impurities are easily removed during manufacture. Less wick impurities = less gunk forming on coils.
3. Rayon cellulose chains are the same as cotton cellulose chains, but theyre shorter, so theres more of them per gram of rayon compared to cotton!
So what you say? Shorter fibres?
If we take a steel chain and cut it in half, we get two lengths, but each length has an end with a broken or open link. Think of this open link as a hydrophilic group. Hydrophilic means it is attracted more to water and molecules like PG and VG which have properties similar to water.
Instead of having a 30ft long chain, we break it into 10x 3ft long chains. Now we have 20 chain ends, where before we had just 2. These chain ends are all the same, more or less.
More chain ends = more "hydrophilic" or water/PG/VG affinity. Thus, the shorter rayon chains are more effective at "sucking up" that PG/VG juice, and you can drip more juice onto the rayon because it adsorbs more PG/VG than an equivalent bundle of japanese cotton.
Now all along the chain of cellulose there are hydrophilic groups, otherwise cotton would behave more like wool and not be effective at adsorbing water. But there is a higher concentration of hydrophilic groups on the end of each cellulose chain or fibre strand. As I said, more fibre/chain ends = more attraction of VG/PG (and water too) into that cellulose fibre than with ordinary cotton.
I wanted to attach a video of the vicose solution polymerizing as it hits the sulfuric acid to form strands, but it wont let me attach it. You can watch it here on wiki:
Rayon - Wikipedia, the free encyclopedia
I am eager to try this stuff out. Better living, through science.
Oh and make sure you dont buy the cheapest of the cheap rayon. It is very pure if made properly, but watch out for low grade or industrial grade rayon. Rayon produced for industrial applications, eg carpets and tyres will not have the same purity standards as that used for clothing.
If it isnt snow white, dont use it.
Rayon is a chemically refined, reformed and purified cellulose based polymer. Many have been lauding it as the next big thing for e-cigarettes. I have personally ordered a sh*t ton of it and I am eager to see if it is as good as reported. I only got about a few pages into the main thread, but I didn't see much technical details about why it is good.
What is rayon?
So both cotton and rayon are cellulose based polymers. As seen in the rayon thread, they have the same basic structure. Celluse is a polymer (like plastic, rubber and proteins).
Like other biological polymers, cellulose is made of monomer units. These are called D-Glucose. You can buy this monomer in the supermarket; its called dextrose. Proteins have various amino acid monomers. Think of monomers as lego blocks for building (the polymer); a big lego tower.
Cellulose has many d-glucose units linked end to end. These form cellulose chains. However, the complex chemical treatment process used for rayon changes things. It breaks longer cellulose chains into shorter elements. These can then be reformed using chemical treatment to form longer or shorter fibres, depending on the application.
How is Rayon made?
Rayon was first commercialized around 1905 as a cotton replacement. Whats wrong with cotton you ask? Rayon can be produced from cheaper precursor materials (eg wood and plant wastes) and specifically tailored for softness, strength and durability depending on application.
I will outline the modern rayon production method briefly. Basically, cellulose fibres are dissolved in chemicals, then pumped through thin jets into a chemical bath where they precipitate as fibres. These fibres are washed, dried, and stretched to straighten them.
1. Wood pulp sourced cellulose is partially dissolved in sodium hydroxide (caustic soda). This does not break the cellulose into its monomer glucose,but breaks up long cellulose monomer chains. Think of this as a chemical treatment to "break up" the fluffy, long filaments of the raw cellulose.
2. The resulting, shorter chain cellulose mush is pressed, dried and crumbled into white cellulose "crumbs". The crumbs are aged in air to help with the de-polymerization of long cellulose chains.
3. The alkali cellulose crumbs are reacted with carbon disulfide (this stuff is dangerously flammable) to form cellulose xanthate. This further de-polymerises the cellulose monomer chains. This carbon disulfide step makes the cellulose even more water soluble by forming soluble xanthate groups on cellulose monomer chains.
4. The cellulose xanathate is aged which further depolymerizes the long cellulose chains. The result is a solution of Viscose, where all the cellulose is fully dissolved and no solids remain.
5. The viscose solution is then pumped through a shower-head like "spinneret" and into sulfuric acid solution, where the dissolved smaller cellulose chains instantly solidify into fibres of rayon.
As can be seen in ray2.PNG(from the main rayon thread on forum), the basic monomer construction of rayon is identical to cellulose. Ok, so if their cellulose chains are identical, then why is rayon so much better??
Why is rayon better?
I have some ideas to explain this:
1. Rayon has a more organized polymer structure because the cellulose in it was reformed into desirable fibre lengths. Think of comparing a big jumbled mess of cotton string to a braided cotton rope made of many cotton twine strands. A cotton rope will soak up more water and remain saturated better than a disorganised cotton string.
2. Rayon is more chemically pure than cotton. Solid cellulose from wood fibres or plants is completely dissolved to form a solid-less solution. This provides a very high purity rayon fibre as impurities are easily removed during manufacture. Less wick impurities = less gunk forming on coils.
3. Rayon cellulose chains are the same as cotton cellulose chains, but theyre shorter, so theres more of them per gram of rayon compared to cotton!
So what you say? Shorter fibres?
If we take a steel chain and cut it in half, we get two lengths, but each length has an end with a broken or open link. Think of this open link as a hydrophilic group. Hydrophilic means it is attracted more to water and molecules like PG and VG which have properties similar to water.
Instead of having a 30ft long chain, we break it into 10x 3ft long chains. Now we have 20 chain ends, where before we had just 2. These chain ends are all the same, more or less.
More chain ends = more "hydrophilic" or water/PG/VG affinity. Thus, the shorter rayon chains are more effective at "sucking up" that PG/VG juice, and you can drip more juice onto the rayon because it adsorbs more PG/VG than an equivalent bundle of japanese cotton.
Now all along the chain of cellulose there are hydrophilic groups, otherwise cotton would behave more like wool and not be effective at adsorbing water. But there is a higher concentration of hydrophilic groups on the end of each cellulose chain or fibre strand. As I said, more fibre/chain ends = more attraction of VG/PG (and water too) into that cellulose fibre than with ordinary cotton.
I wanted to attach a video of the vicose solution polymerizing as it hits the sulfuric acid to form strands, but it wont let me attach it. You can watch it here on wiki:
Rayon - Wikipedia, the free encyclopedia
I am eager to try this stuff out. Better living, through science.
Oh and make sure you dont buy the cheapest of the cheap rayon. It is very pure if made properly, but watch out for low grade or industrial grade rayon. Rayon produced for industrial applications, eg carpets and tyres will not have the same purity standards as that used for clothing.
If it isnt snow white, dont use it.
Attachments
Last edited: